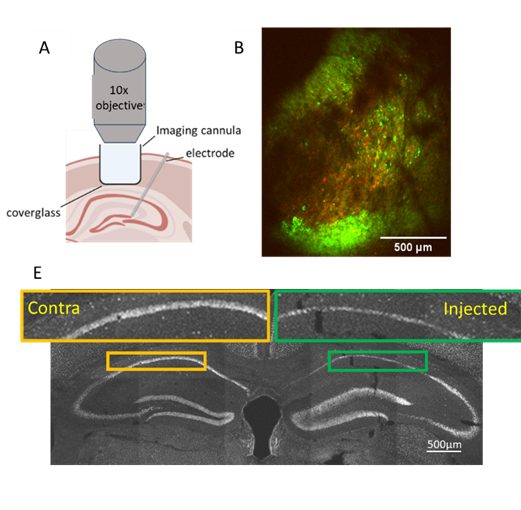
In most patients with epilepsy, the brain produces predominantly normal rhythms until the paroxysmal event of a seizure occurs. What is happening at the cellular level as the brain transitions to the crippling state of seizure? Characterizing, at a detailed level, the nature of ictogenesis will provide insight into how we might reduce the probability that the brain tips into this pathological state. In an ongoing collaboration with Lauren Lau, we are imaging neuronal activity with cellular resolution, using in vitro and in vivo mouse models of epilepsy to address fundamental questions about what patterns of neuronal activation lead to seizure onset. We have found that, even in highly reduced models of epilepsy, although seizures emerge from a consistent brain region, seizure onset is stochastic at the single neuron level.

We are currently advancing this study to look at ictogenesis in vivo as spontaneous seizures emerge in the intrahippocampal kainate model of epilepsy. We maximize the likelihood of imaging the seizure focus by implanting an imaging cannula with a large field of view directly over the side of kainate injection.